Testing the Effectiveness of Naturally-Sourced Antimicrobial Compounds (TENAC)
Table of Contents
Natural Sources of Antibacterial Compounds
 Antimicrobial resistance (AMR) is one of the most prevalent worldwide public health concerns. The true world burden of AMR is unknown, however, in the United States AMR proves to be fatal in approximately 35,000 people annually and impacts the treatment regime of 2.8 million people.1 In the United States, this results in a higher number of serious infections, increased costs in second-line drugs, and extended hospital admissions.2 With the current trajectory it is estimated that by 2050 AMR will cost upward of 100 trillion USD and claim 10 million lives a year.3
Antimicrobial resistance (AMR) is one of the most prevalent worldwide public health concerns. The true world burden of AMR is unknown, however, in the United States AMR proves to be fatal in approximately 35,000 people annually and impacts the treatment regime of 2.8 million people.1 In the United States, this results in a higher number of serious infections, increased costs in second-line drugs, and extended hospital admissions.2 With the current trajectory it is estimated that by 2050 AMR will cost upward of 100 trillion USD and claim 10 million lives a year.3

While AMR happens naturally as bacteria adapt to challenging environments, medical misuse, agriculture dependence, and increases in travel, all contribute.2 Of note, six opportunistic pathogens have been recognized as a significant health risk by the Infectious Disease Society of America.4 These multidrug-resistant Gram-positive and Gram-negative bacteria are known as the ESKAPE pathogens, an acronym for Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species.
Although many new antimicrobials have been discovered since the introduction of penicillin in the 1940s (see below), the cost of investment (approximately 1.5 billion USD) and return on that investment (46 million USD per year) has meant many pharmaceutical companies no longer perceive a cost-benefit ratio in research and development. Currently, only four major pharmaceutical companies maintain an active antibiotic research program.5 Furthermore, as the COVID-19 pandemic has shown, the world is not prepared for the threat of infectious diseases, so the development of new antimicrobials is critical. Many antimicrobials are based upon novel compounds derived from nature. Therefore, the focus of my research is on assessing the effectiveness of chemicals isolated from terrestrial plants and soil microorganisms.
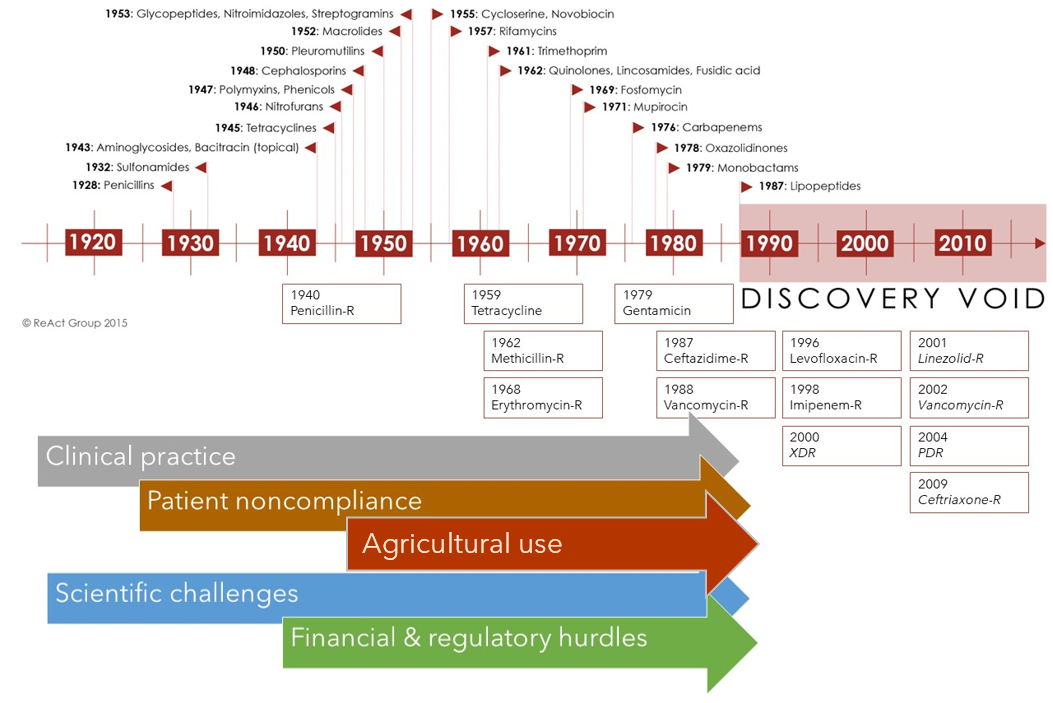
Contributing factors to antibiotic discovery void. H.Walter, adapted from: Reactgroup
Bioactive Molecules from Plants

Baccharis pilularis

Salvia spathacea
Historically, over 5000 Northern American flora were used for medicinal purposes by its Indigenous peoples.6–8 Many continue to be used today by these same communities. In the laboratory, antimicrobial properties have been demonstrated in some of these species. For example, extracts of Umbellularia californica (California Bay Laurel) inhibits the growth of some Gram-positive bacterial species, including antibiotic-resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA).9 While Salvia apiana (White Sage) is effective against various Gram-positive and Gram-negative bacteria, including Klebsiella and Staphylococcus.10 Despite the wide use by Indigenous communities, the clinical properties of other species remain poorly elucidated. These include Baccharis pilularis and Salvia spathacea. The leaves of S. spathacea, also known as Hummingbird Sage or Pitcher Sage, have historically been used as a decongestant and treatment by the Chumash people.11,12 Baccharis species have been used medicinally by many Indigenous peoples. Historical documentation shows the Indigenous peoples of the Greater San Francisco Bay area used B. douglasii as a disinfectant and B. pilularis as a panacea.13,14 Although Bocek13 states this use was by the Costanoan/Ohlone people, we now know there are many local Indigenous people including the Me-Wuk, Ramaytush and Chochenyo people which may have used these same medicinal herbs. In our experiments, oils are extracted, purified, then assessed against safe-ESKAPE-relatives for antibiotic potential.
Bioactive Molecules from Soil
Soil is a rich ecosystem with diverse microbial life, and resident microorganisms must adapt to changes in both biotic and abiotic conditions, often by producing a wealth of molecules that inhibit competing microbes. Antimicrobials, therefore, provide an advantage in competing for food and other limited resources. Historically, soil has provided a wealth of antimicrobial compounds. Almost two decades after penicillin’s discovery, streptomycin was isolated from Streptomyces griseus,15 a soil-dwelling organism. This was quickly followed by chloramphenicol (1947), tetracycline (1948), neomycin (1949), and vancomycin (1950). However, the soil is a heterogeneous environment, where multiple species co-exist. For example, Waksman processed 10,000 samples before identifying streptomycin.16 Thus, isolating new antibiotics from soil microbes is a significant challenge.
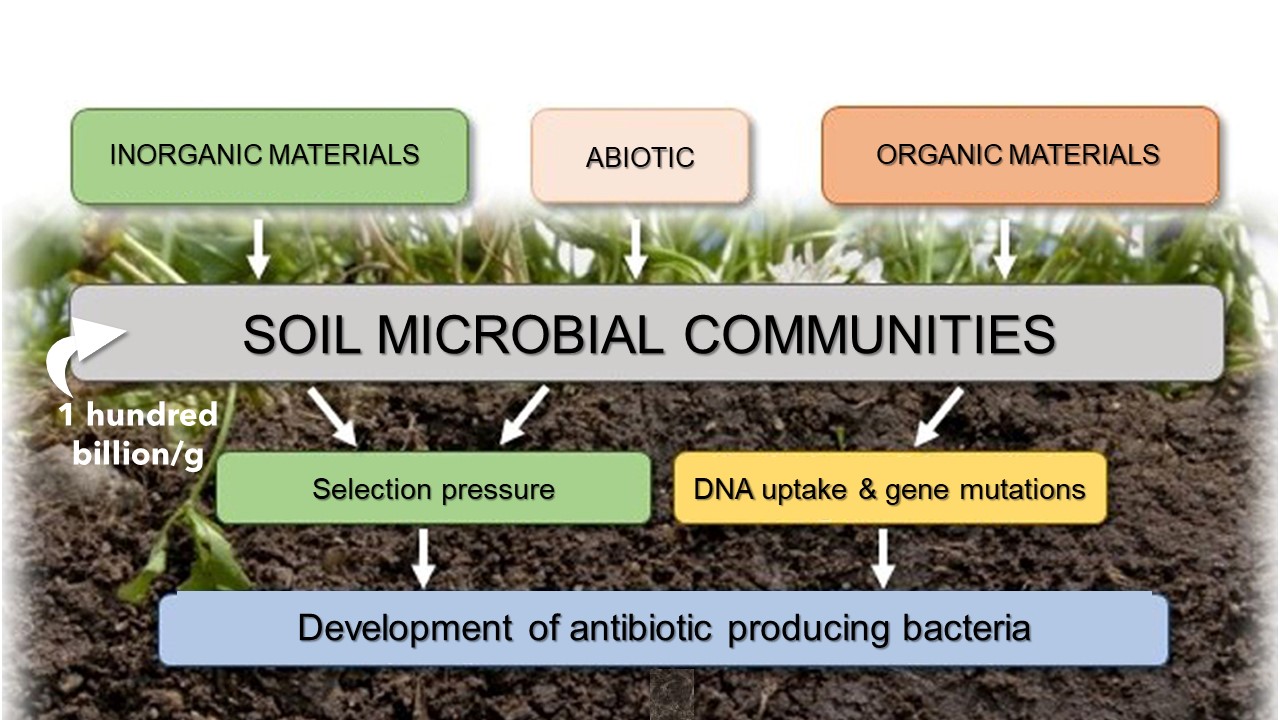
Environmental Evolutionary Pressures on Soil Microorganisms. H. Walter, adapted from Cycoń et al., 2019
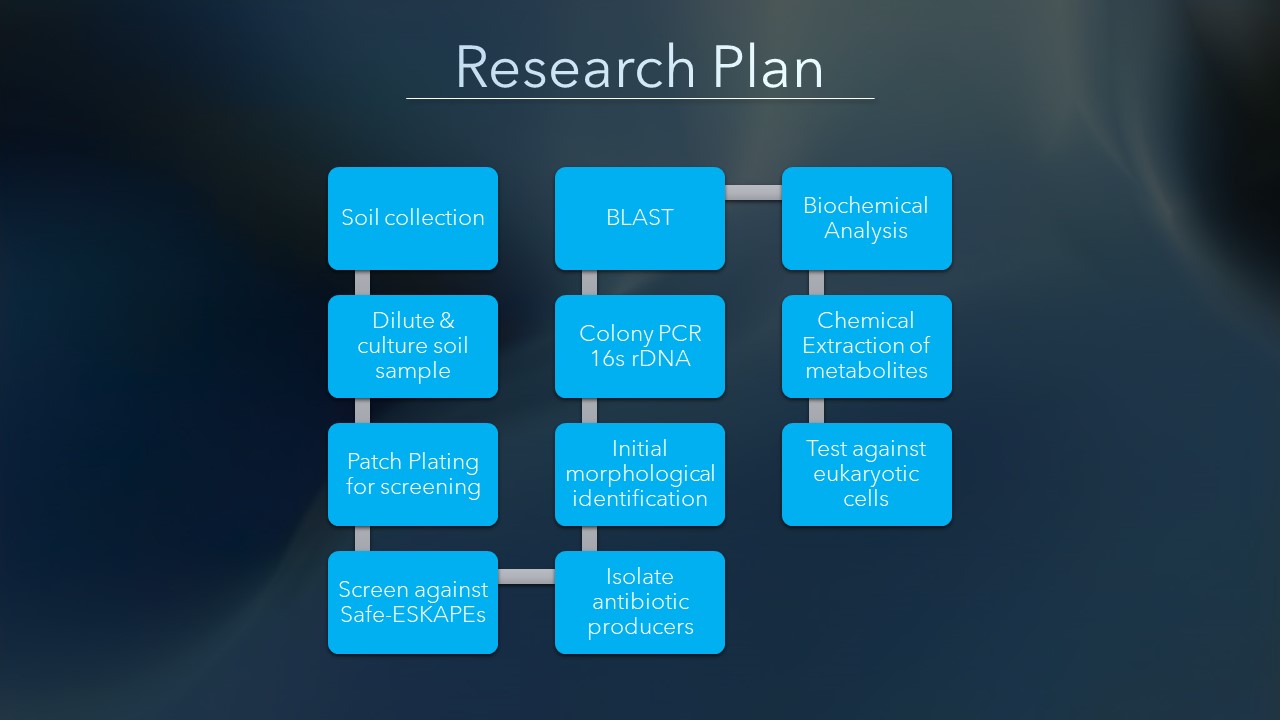
Research Plan
My Research Team
I am a new faculty member at Clark Atlanta University. Previously, I taught Microbiology and Immunology for 18 years at Mills College, and Northeastern University. I am passionate about my subject. My favorite topic to teach is metabolism, something I struggled with as an undergraduate, and I enjoy seeing my students understand it as I never did. I have been a member of the Small World Initiative since 2018, and a Principal Investigator for them since 2021.
First-year and second-year students in my laboratory can follow a Course-Based Undergraduate Research Experience (CURE). CUREs support the retention of students and offer early opportunities to engage in research. Those with microbiology experience can omit the CURE experience, and often become mentors in their senior year. The following students have been members of my research group:

References
- Centers for Disease Control and Prevention (U.S.). Antibiotic resistance threats in the United States, 2019. (2019).
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 12, 3903–3910 (2019).
- O’Neill, J. Tackling drug-resistant infections globally. (2016).
- Boucher, H.W. et al. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48, 1–12 (2009).
- Plackett, B. Why big pharma has abandoned antibiotics. Nature 586, S50–S52 (2020).
- Stockwell, C. Natureʾs pharmacy: a history of plants and healing. (Century, 1988).
- Borchers, A.T. et al. Inflammation and Native American medicine: the role of botanicals. Am. J. Clin. Nutr. 72, 339–347 (2000).
- Moerman, D.E. An analysis of the food plants and drug plants of native North America. J. Ethnopharmacol. 52, 1–22 (1996).
- Carranza, M.G. et al. Antibacterial activity of native California medicinal plant extracts isolated from Rhamnus californica and Umbellularia californica. Ann. Clin. Microbiol. Antimicrob. 14, 29 (2015).
- Afonso, A.F. et al. The Health-Benefits and Phytochemical Profile of Salvia apiana and Salvia farinacea var. Victoria Blue Decoctions. Antioxidants 8, 241 (2019).
- Timbrook, J. Chumash ethnobotany: plant knowledge among the Chumash people of southern California. (Santa Barbara Museum of Natural History; Heyday Books, 2007).
- Dye, C. & Crane, A. California Native Medicinal Plants. (2017).
- Bocek, B.R. Ethnobotany of Costanoan Indians, California, based on collections by John P. Harrington. Econ. Bot. 38, 240–255 (1984).
- BRIT – Native American Ethnobotany Database. http://naeb.brit.org/.
- Schatz, A. et al. Streptomycin, a Substance Exhibiting Antibiotic Activity Against Gram-Positive and Gram-Negative Bacteria. Proc. Soc. Exp. Biol. Med. 55, 66–69 (1944).
- McKenna, M. Hunting for Antibiotics in the World’s Dirtiest Places. The Atlantic.